Lentiviral vector-mediated gene therapy to treat hearing and balance loss patients
Project partner:
Prof. Dr. Michael Morgan
Dr. Juliane Schott
Prof. Dr. Hildegard Büning
John W. Hadden II, CEO of ViroCell Biologics Ltd. (London, UK)
Prof. Farzin Farzaneh, CSO of ViroCell Biologics Ltd. (London, UK)
Collaboration partners:
Prof. Dr. med. Athanasia Warnecke, Medizinische Hochschule Hannover
Prof. Dr. Hinrich Staecker, University of Kansas (USA)
Hearing loss is the most common sensory impairment in humans, with serious consequences both for the individuals affected and for society. A substantial proportion of cases of hearing loss is caused by genetic mutations. For some of the affected genes, the underlying mutation not only causes hearing loss, but leads to syndromes of hearing loss in combination with other dysfunctions, e.g. of the sense of balance.
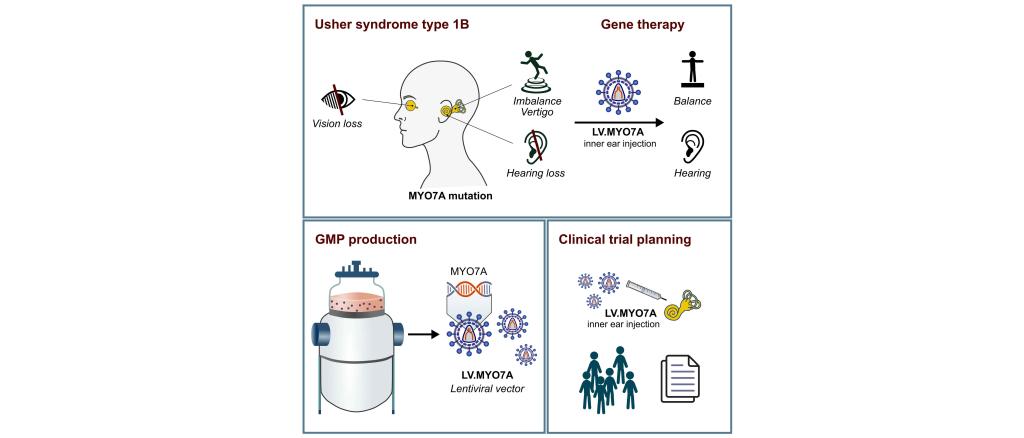
Usher syndrome type 1B (USH1B) is one of the most severe hearing loss syndromes and is caused by mutations in the MYO7A gene. USH1B patients suffer from congenital deafness combined with severe vertigo and progressive blindness that begins in adolescence. Current treatment options are limited to hearing aids and cochlear implants, which provide only a temporary or partial solution to hearing loss and are not suitable for all patients. None of the currently available therapeutic options addresses balance loss or blindness.
Prof. Schambach's team has developed a novel gene therapy approach to simultaneously treat hearing and balance loss. The approach is based on lentiviral vector particles that are used as biological vehicles to introduce a correct, functional variant of the MYO7A sequence into the cells of the inner ear, which contains the auditory and vestibular organs. This approach has already shown promising results in mouse models, with positive effects on both hearing and the sense of balance in animals with a Myo7a mutation.
In this project funded by ForTra, the clinical translation of this new gene therapy approach will be advanced to provide a treatment option for patients with hearing and balance loss caused by MYO7A mutations. In particular, the funding will enable the production of the therapeutic viral vector particles under GMP conditions for a future first clinical trial, and will include the actual planning of the trial.
Here you can find further information.