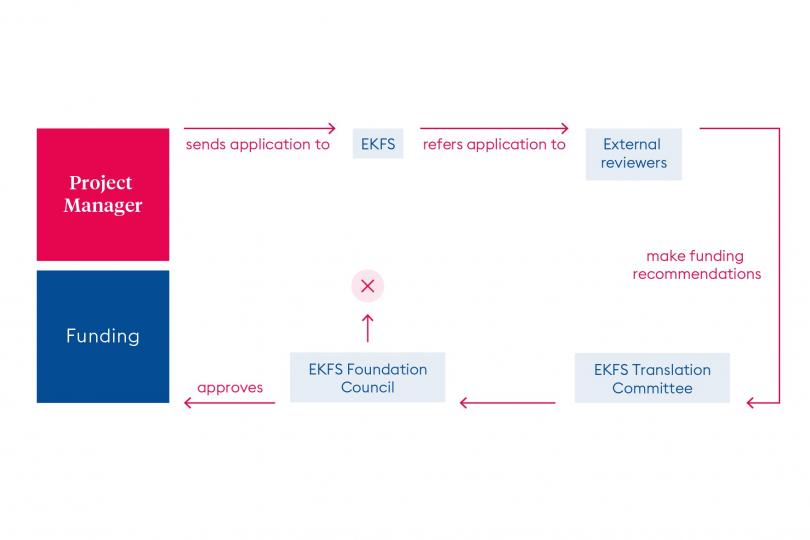
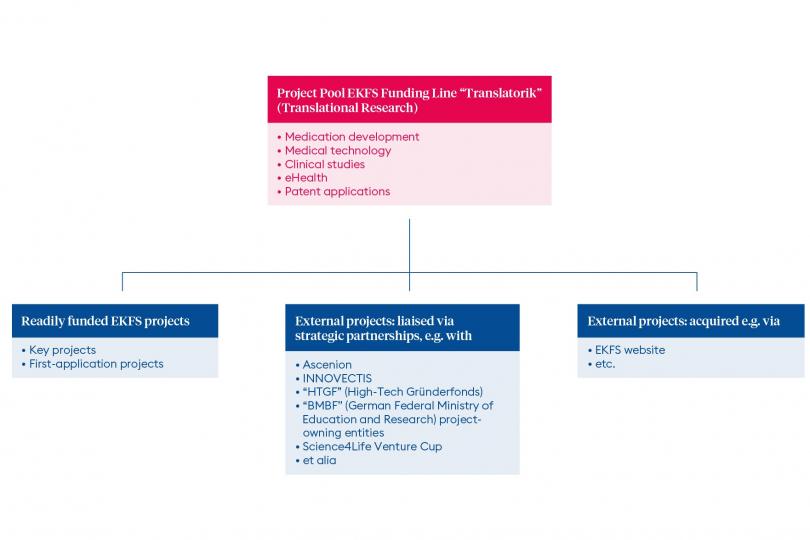
An overview of the projects funded from the funding line “Translational Research” can be found here.
There is a wide gap in Germany between successful fundamental medical and scientific research and its real-time, direct application in routine clinical practice for the benefit of patients. This is due, among other things, to the scarcity of funding for gathering the necessary preclinical data and for initial clinical trials. In the case of therapeutics that require a considerable amount of investment to bring them to market, for example, financially sound investors are often only interested in financing further product development with risk capital after the successful completion of clinical phase 1 and 2 trials.
The EKFS’s aim through its non-profit subsidiary ForTra gGmbH für Forschungstransfer is to fund and actively support research projects, in order to bring promising developments to the application stage as quickly as possible to help the sick or prevent diseases. The translational research funding line is open to all patient-side applications, regardless of market size.
Funding Programme
The translational research funding line is aimed to support innovative projects with high translational potential, for which the respective patient-related application potential is clearly defined and for which the first convincing research findings have already been demonstrated. The EKFS supports translational research projects by funding of key experiments or studies that are required to validate the medical approach or the product (drug). In addition, projects that are funded from the translational research funding line have the possibility to receive close expert support for project development and technology and knowledge transfer into application.
Applicants can apply for validation and advancement of their project, for example by payment for external research services, such as
- a toxicity study, large animal model, etc.
- financing of further validation studies in cohorts for a new clinical diagnostics
- start-up financing for an initial clinical trial (e.g. “first-in-human” trial)
As most application-oriented projects are at different stages of development and require very different measures to advance them, the projects approved within the scope of the translational research funding line can receive further support via individually tailored support measures, e.g.:
- financing of consulting service, e.g. from a patent attorney or with regard to regulatory matters
- financing of costs to patent promising inventions
- coverage of costs for professional advice on project development and on technology and knowledge transfer of the relevant technology or innovation by an external advisor officially accepted by ForTra
The subsequent continued financing of an advanced-stage translational project previously supported by ForTra, for example by an industrial partner, is encouraged. One exception is direct or indirect (co-)financing of the development work of a commercial partner, who, for example, can claim property rights of the product to be developed. The research institute of the principal investigators (contract partner of ForTra) has to keep full FTO (freedom-to-operate) for commercial exploitation during the time of ForTra funding.
The main applicants are academic scientists, who can send an outline of their project to ForTra at any time. The recipient of subsequent funding is the non-profit research facility in Germany that employs the applicants. No funding agreements may be concluded with readily established business firms.
The sample funding contract currently used for the Translational Research Funding line can be found below for general information (download).
Application submission
Electronic application submission via email addressed to antrag@fortra-forschungstransfer.de is possible at any time and does not require a specific format template. What is required:
- the actual application itself
- the filled-out Assessment Form for the “Translatorik” (‘Translational Research’) line of funding
- the signed and scanned consent of the applicants for data storage
Both documents (Assessment Form and consent) can be downloaded further down on this website.
A number of details regarding application submission can also be found among the answers to the “Frequently Asked Questions (FAQs)” on the home page. Making contact with Prof. Zörnig by telephone prior to application submission is recommended in order to clarify fundamental funding capability for the project along with the particulars involved towards application submission.
Frequently Asked Questions (FAQs)
Application submission is possible at any time. There are currently no time limits or deadlines. Processing commences directly following receipt of the application.
Alongside the application itself, applicants must attach and include the completely filled out “Translation Assessment Form” from EKFS for the funding line “Translational Research” and the signed and scanned consent of the applicants for data storage.
Both forms can be downloaded from the EKFS homepage.
The official funder of projects is ForTra gGmbH für Forschungstransfer, a non-profit subsidiary at the Else Kröner-Fresenius-Stiftung (EKFS) foundation. The limited-liability company concludes contracts with the respective employers of project directors. In other words, the funding recipients are academic research facilities (hospitals, research institutes, etc.) or other non-profit scientific/scholastic institutions.
There is no template for applications which, however, ought to include the following parts:
- A summary of the project, including indication of the funding sum being applied for, the term of the project and the designated object of funding
- An overview regarding the field that a non-expert interested in science is able to understand
- Own preliminary work, with the original data of relevant results/experiments (also including: what is the actual status of own project-related patent families? How far has the project advanced at the timepoint of submission of the application?)
- A precise indication regarding the amount of funding resources being applied for along with a precise portrayal of what they are supposed to be utilised for (work programme)
- A detailed depiction of how the project is supposed to be advanced after the funding from ForTra ends – Who is able to or shall make funds available following the funding from ForTra? Do investors exist who intend to invest money in the project upon achievement of certain “milestones” once the funding agreement has expired?
A maximum sum for funding has not been established. Until now the average funding sum has amounted to around EUR 300,000, but in the past projects involving either higher or lower project costs have also been supported. The co-financing of projects together with another sponsor is equally possible. The foundation assumes only those types of project costs that applicants are unable to finance via other organisations and/or investors.
The potential terms for projects that can be applied for are flexible. Many projects are funded for 2 or 3 years, but depending on the respective work programme both shorter and even longer terms are possible.
On average it takes roughly 3 months from the day the foundation receives the completed application until the final funding decision.
At the present time, the non-profit company ForTra GmbH für Forschungstransfer at EKFS does not conclude any funding agreements with readily existing firms/spin-offs, only with academic research facilities (hospitals, research institutes, etc.) or other non-profit scientific/scholastic institutions (in other words, with the project directors’ employer).
The sample funding contract (including the “Mitarbeitervereinbarung”, only available in German) can be downloaded from this site. It is not negotiable, and applicants should check with their legal office/technology transfer office whether it will be acceptable in its current version for their research facility.