Precision immunotherapy: Mutation-specific TCR-T cell therapy for MyD88L265P mutated lymphoma – GMP-grade vector production and establishment of TCR-T cell manufacturing
Project partner:
Charité-Stem Cell Facility, Charité - Universitätsmedizin Berlin, Campus Virchow Klinikum
Prof. Dr. rer nat. Gerald Willimsky, Institut für Immunologie - Charité-Universitätsmedizin Berlin und Deutsches Krebsforschungszentrum (DKFZ)
Dr. rer. nat. Felix Lorenz, Max-Delbrück-Centrum für Molekulare Medizin (MDC)
Project:
40% of patients with newly diagnosed diffuse large B-cell lymphoma (DLBCL, the most frequent subentity among high grade lymphomas) fail to respond to primary therapy or relapse (r/r DLBCL). Most of these patients cannot be cured by either salvage chemotherapy followed by autologous stem cell transplantation or by CD19-CAR T-cell therapy. The prognosis is particularly poor for patients with primary central nervous system DLBCL (PCNSL) who relapse after the highly aggressive primary treatment or for the many elderly / unfit / frail patients who cannot undergo the intensive treatment needed for cure. New effective strategies for the curative treatment of r/r DLBCL and PCNSL are therefore urgently needed.
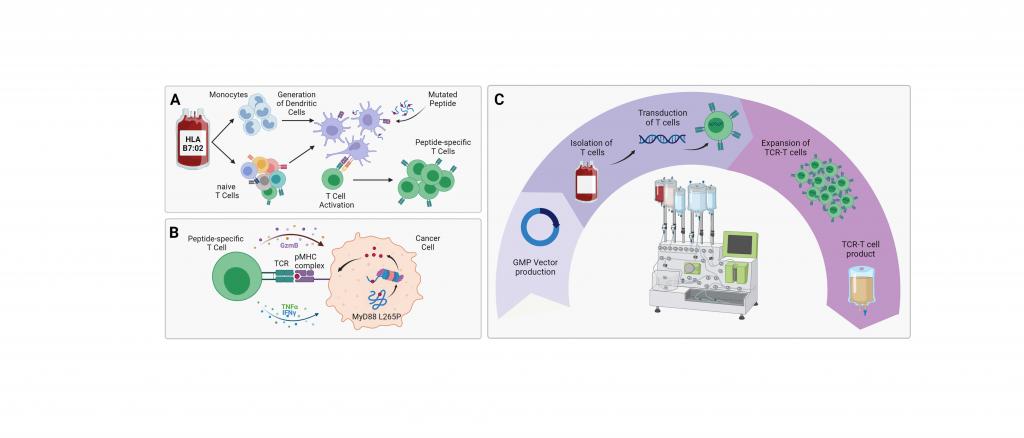
We have developed a personalized T-cell therapy using a human T-cell receptor (TCR) targeting the lymphoma-specific driver mutation MyD88L265P. This mutation is frequently found in DLBCL lymphomas, in particular in almost half the cases of PCNSL. According to the high specificity of this mutation found exclusively in tumor cells, our TCR only recognizes and kills lymphoma cells but not normal tissue. The tissue histocompatibility antigen HLA-B7 must be expressed by the cells of the host so that the TCR can attack lymphoma cells. Around 15-20% of the European population bears the HLA-B7 antigen. We were able to show the effectiveness of our TCR-based approach both in vitro and in vivo in xenograft experiments.
The successful translation of this precision immunotherapy into the clinic would not only bear the potential for long-term remission in a deadly disease, but would also represent a significant improvement in the “benefit/side effect ratio” compared to classic immunochemotherapy and CAR-T cell therapy due to its tumor cell specificity.
We are planning a prospective, single-arm multicenter phase I study in HLA-B7 positive patients with r/r MyD88-L265P mutant DLBCL/PCNSL. The ForTra gGmbH is funding the “good manufacturing practice” (GMP) grade production of the gene vector needed for the transfer of the tumor-specific TCR into the patients´ own T lymphocytes, plus the establishment of the manufacture of the TCR-T cell products.
Here you can find further information.




