Pleiotropic effects of Empagliflozin in human and mouse myocardium

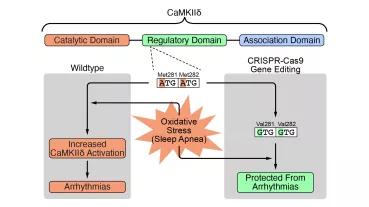
Empagliflozin is an inhibitor of the sodium-dependent glucose co-transporter 2 (SGLT2) and is clinically approved as an oral antidiabetic drug. By inhibiting SGLT2 in the proximal tubule of the kidney, empagliflozin reduces the reuptake of glucose and sodium and thereby lowers blood glucose. In the EMPA-REG OUTCOME trial, empagliflozin was evaluated for its cardiovascular safety. The results showed that empagliflozin reduced cardiovascular mortality, all-cause mortality and heart failure hospitalization rates. Possible direct effects of empagliflozin on the human heart that may explain or contribute to the clinical observations remain unknown. Therefore, the aim of the present study was to assess the effect of empagliflozin on myocardial function in the human and murine myocardium and to identify the underlying mechanisms of action (together with PD Dr. Streckfuß-Bömeke).
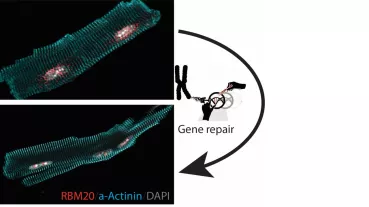
In this project a comprehensive investigation of the diabetic independent effects of Empagliflozin on myocardial contractility, ion homeostasis, and electro-mechanical coupling will be performed. We are going to use isolated cardiomyocytes and multicellular myocardium from freshly explanted human failing hearts as well as induced pluripotent stem cell-derived cardiomyocytes. In these settings, we will perform acute drug treatment as well as chronical treatment reflecting the EMPA-REG OUTCOME trial time range. In addition to the functional investigations, we will fundamentally explore molecular changes in empagliflozin-treated cardiomyocytes including a Next Generation Sequencing (NGS) approach. Based on the results of these whole exome findings we will investigate specifically altered signal cascades in order to reveal the underlying effect of Empagliflozin on the heart. Thus, this study may establish a rationale for further translational studies to investigate a diabetes-independent role of empagliflozin in patients with cardiac disease and, in particular, with heart failure.