
EKFS has selected six Else Kröner Memorial Fellowship recipients who will implement a promising medical research project as a result of being released from clinical responsibilities for a period of two years. Each scholarship is endowed with EUR 230,000.
The following scientists are receiving a Memorial Fellowship this year:
Dr. Michael S. Balzer, Department of Nephrology and Medical Intensive Care, Charité – University Hospital
Project: Endophenotypes of kidney disease progression at the single-cell level
Despite the kidney’s tremendous capacity for renal functional reserve (RFR), acute kidney injury (AKI) often leads to chronic kidney disease (CKD). Over 850 million people are affected worldwide. There is a lack of medication potentially able to prevent or stop a deterioration of renal function following acute damage. Modern single-cell transcriptomic technologies bear the potential to fundamentally change our knowledge about disease processes at the cellular level. The project aims to elucidate cellular signatures (“endophenotypes”) of both successful and maladaptive/scarring repair. Its target is to revolutionize classical paradigms of how kidney disease is characterized and consequently lay the groundwork for the development of putative targeted therapies for AKI.

Dr. Julius C. Fischer, Department of Radiation Oncology, Technical University of Munich (TUM)
Project: Influence of hormonal regulatory circuits on antitumor immune responses following radiation therapy
The junior research group is investigating mechanisms to improve therapeutic response rates following radiation therapy or combined radioimmunotherapy. At the same time, the team is researching the underlying mechanisms of the side effects of such combination therapies. In this experimental research project the scientists want to investigate the influence of hormonal mechanisms on the development of antitumor immune responses following radiation therapy, or the combination of radiation therapy with immune checkpoint blockade. The project is based on the hypothesis that radiation therapy activates specific hormonal circuits that influence such antitumor immune responses. This knowledge could be used in the future to optimize cancer therapies.
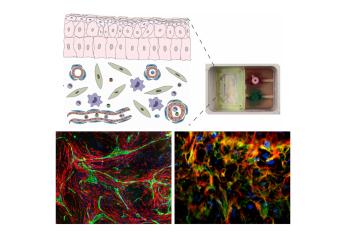
Dr. Alexandru-Emil Matei, Clinic for Rheumatology and Hiller Research Center, University Hospital of Düsseldorf and Heinrich-Heine-University (HHU) Düsseldorf
Project: Advanced engineered organotypic models of fibrosis for pathophysiological studies and for testing novel therapeutic approaches
Fibrotic remodeling processes that occur for example in systemic sclerosis are a common cause of morbidity and mortality in industrialized countries. The currently used models of fibrosis for therapeutic testing are based on mice or cultured fibroblasts, and thus reproduce the development and course of fibrotic diseases only to a limited extent. In the proposed project the scientists aim to establish complementary models of fibrosis using human cells with varying complexities and throughput capacities. These models are intended to reconstruct the emergence and progression of fibrotic diseases better than conventional models, and consequently be able to provide higher predictive performance for the use of antifibrotic substances in human beings.

Dr. Julien H. Park, Department for Children and Adolescents – General Pediatrics, Münster University Hospital
Project: N-acetylmannosamine as potential therapy for Golgi homeostasis disorders
Congenital disorders of glycosylation (CDG) are inborn errors of metabolism that lead to severe, potentially fatal multisystem diseases. The project studies how cellular damage occurs due to the lack of sialic acid. The effect of the sialic acid precursor molecule N-acetylmannosamine on sugar chains (glycans) and metabolic processes is being investigated in order to develop a therapy on a long-term basis. Due to the key role of hyposialylation in the case of diseases affecting the central nervous system, the knowledge gained can deepen the understanding of these frequent disorders.
Dr. Robert Seifert, Clinic for Nuclear Medicine and Münster University Hospital, Clinic for Nuclear Medicine, University Hospital Essen
Project: Innovative strategies for optimization of randomized therapeutic trials using molecular imaging and artificial intelligence
The prostate-specific membrane antigen (PSMA) is overexpressed in prostate cancer cells and can be utilized as a target structure to treat patients with advanced prostate cancer. PSMA therapy can display a high efficiency and prolong patients’ survival time; however, it is currently not clear which patients will benefit from therapy and which will not respond to it. This proposal therefore aims at optimizing the selection process for PSMA therapy by means of methods of artificial intelligence, characteristics of all metastases, and additional features. The findings can improve the understanding of prostate cancer. Furthermore, the methods developed via this proposal can equally be used to improve the image-based inclusion criteria of other cancerous entities which receive precision anti-cancer treatments.
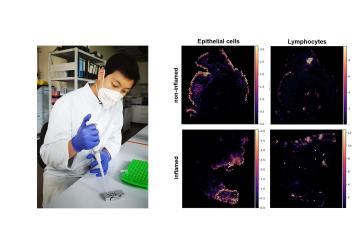
Dr. Florian Tran, Department of Internal Medicine I & Institute of Clinical Molecular Biology, UKSH Kiel
Project: Spatial and longitudinal multi-omics profiling to decipher remission under biological therapy in the case of ulcerative colitis
Ulcerative colitis (UC) is an incurable chronic intestinal disease which can severely reduce the quality of life for those affected. Even though novel therapy options are available, a long-term remission (absence of symptoms and inflammation) cannot be achieved in a large proportion of UC patients. In this project modern sequencing technologies will be utilized to detect complex molecular signatures displaying spatial resolution (spatial multi-omics) in intestinal biopsy samples from UC patients receiving biological therapy. The aim is to identify molecular predictors for long-term remission. This project will therefore contribute to biomarker-based precision medicine for patients suffering from UC.